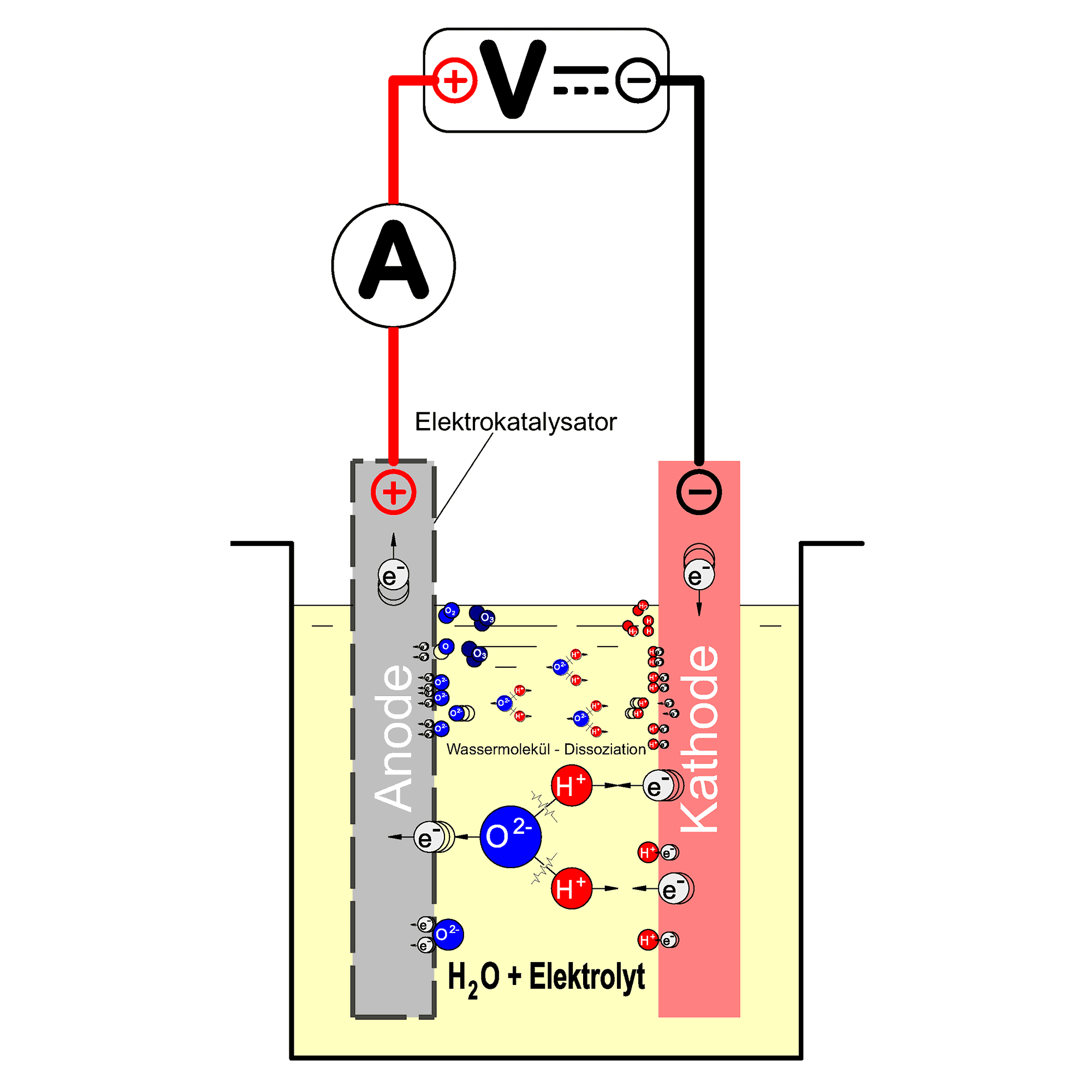
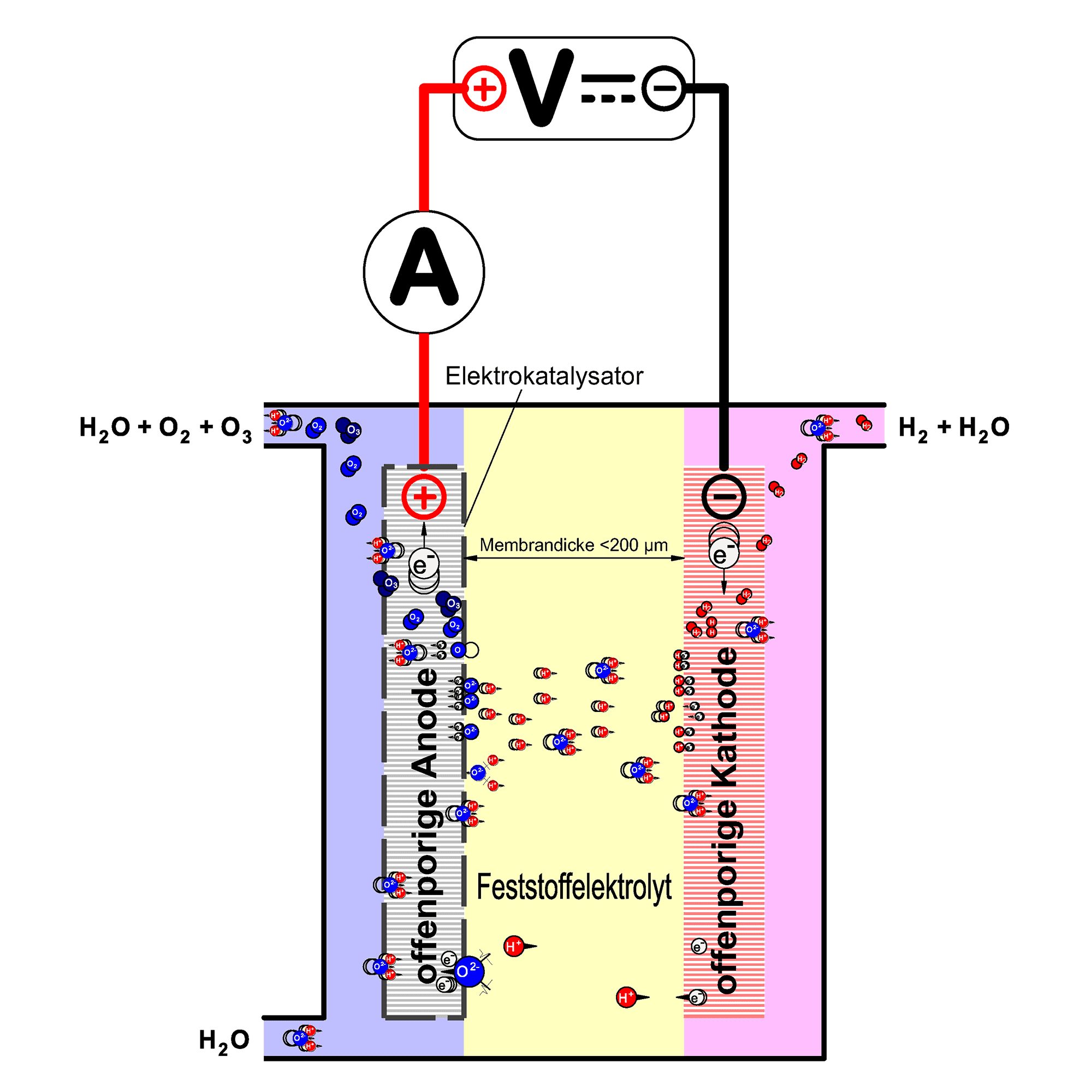
The principle of water electrolysis
Electrolysis can be used to force chemically involuntary processes by performing electrical work. A DC voltage must be applied for the cell reaction to take place.
For water electrolysis, the electrochemical cell consists of two electrodes (anode and cathode), both are immersed in water. The conductivity of the water is increased by adding a little acid, usually sulphuric acid. The electrochemical cell for water electrolysis is shown schematically in the adjacent figure.
If a DC voltage greater than the so-called decomposition voltage of the water is applied to an electrolysis cell, water is broken down into its elements hydrogen and oxygen. The quantity of gases formed is directly proportional to the charge flowing through the cell (Faraday’s law).
The decomposition voltage required for the process corresponds to the value of the chemical energy stored in the products hydrogen and oxygen.
Electrolytic ozone generation
The energy, and therefore the chemical reactivity of the adsorbed oxygen produced as an intermediate product during oxygen evolution, increases with increasing overvoltage. At an electrode potential above approx. 2.3 V, the formation of free oxygen atoms is theoretically possible. Free oxygen atoms are very reactive and can react with molecular oxygen to form ozone. To prefer the formation of ozone under these circumstances, the anode is coated with an electrocatalyst.
Instead of the liquid electrolyte required for water electrolysis, a solid electrolyte (polymer electrolyte membrane, PEM) is used in Innovatec’s ozone cells. This consists of a polymer which has the property of transporting electrical charges.
In this way, it is no longer necessary to artificially increase the conductivity of the feed water. This means that electrolytic ozone generation is also possible in ultrapure water. At the same time, the membrane serves as a separator for the physical separation of the electrolysis products. The ozone electrolysis cell is shown schematically in the adjacent figure.
In this way, only the anodically produced ozone is fed into the water circuit in which the electrolysis cell is installed. The cathodically produced hydrogen is removed via a separate pipe.